Science Bank 3 (Patterns of Reactivity): you just need part 3 (from 8m 50s) covering the reactivity series of metals, displacement & the thermite reaction:
Revision on the Reactivity Series from KS3 Bitesize.
IGCSE Chemistry, a 25m lesson from ChemistryKlipz covers metal reactivity (from 6m 20s to 11m), rusting (to 13m 50s) & displacement rcts (to 18m 30s).
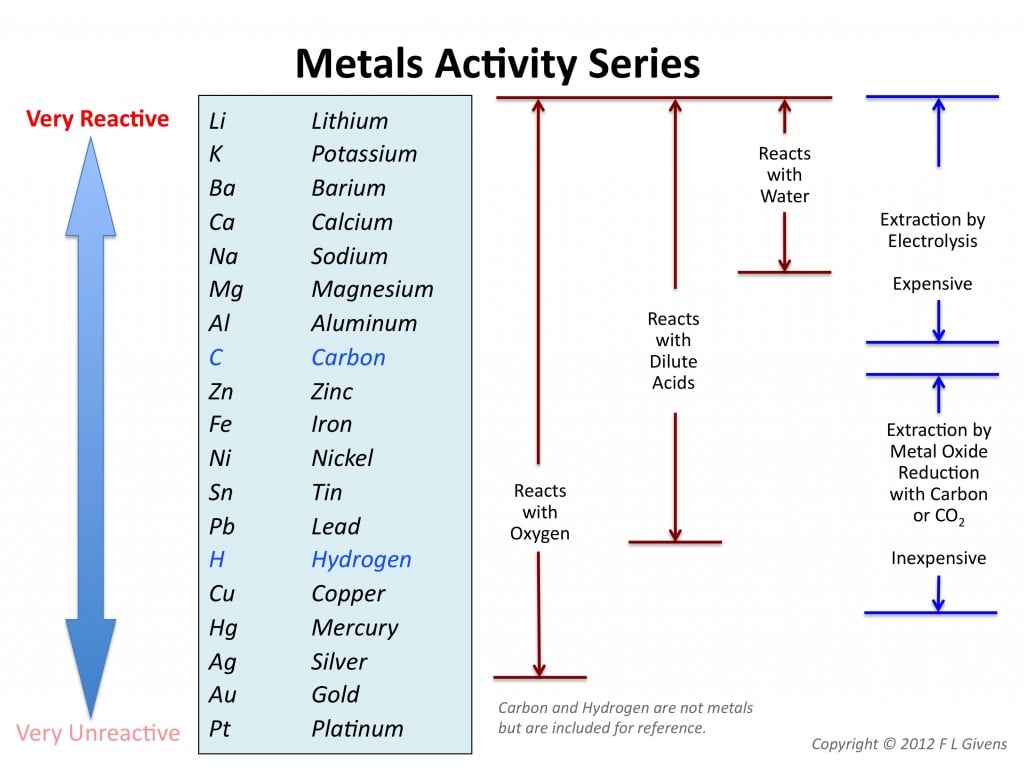
I really like this, but I’m confused why you’ve put lithium at the top, when both sodium and potassium are more reactive? (the alkali metals are more reactive down the group)
Ah.
I really liked this too but did not create it. And clearly I did not look too closely at it. You are right, some of those metals are in the wrong order.
At least it agrees with the IGCSE syllabus:
“Place in order of reactivity: potassium, sodium, calcium, magnesium, zinc, iron, (hydrogen), copper”
Thanks for pointing out the error(s)
wrong information very bad
do not use
it helped